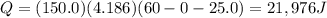CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(...

Chemistry, 22.04.2020 23:16 ethangorrell67
CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(s), has been proposed as a substance that can be used to heat water quickly for portable heating packs or for cooking. When placed in water, Cao(s) reacts as shown by the equation above.
A student wants to design a heating pad that could heat a 150.0 g sample of water from 25.0°C to 60.0°C.
Calculate the amount of heat, in joules, that the water must absorb for its
temperature to change by this amount. (Assume that the specific heat capacity
of the water is 4.18 J/gK).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3

Chemistry, 23.06.2019 14:00
What is the final volume in milliliters when 0.641 l of a 34.0 % (m/v) solution is diluted to 23.5 % (m/v)?
Answers: 1
You know the right answer?
Questions





Mathematics, 26.02.2020 06:02

Biology, 26.02.2020 06:02

Computers and Technology, 26.02.2020 06:02











Biology, 26.02.2020 06:02


 , the amount of heat that must be supplied to the substance must be:
, the amount of heat that must be supplied to the substance must be: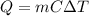
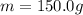 is the mass
is the mass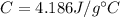 is the specific heat capacity of water
is the specific heat capacity of water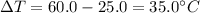 is the increase in temperature
is the increase in temperature