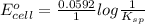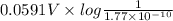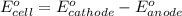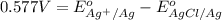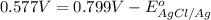Chemistry, 22.04.2020 22:03 Buttercream16
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the solubility product constant for AgCl at 298 K is 1.77 × 10 − 10 and the standard reduction potential of the half‑reaction Ag + ( aq ) + e − − ⇀ ↽ − Ag ( s ) is + 0.799 V .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1
You know the right answer?
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the...
Questions

Mathematics, 29.09.2019 03:30

Physics, 29.09.2019 03:30

Social Studies, 29.09.2019 03:30



History, 29.09.2019 03:30


History, 29.09.2019 03:30


History, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30

English, 29.09.2019 03:30

History, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30

Biology, 29.09.2019 03:30



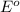 for the half-cell reaction is 0.222 V.
for the half-cell reaction is 0.222 V.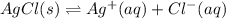
![K_{sp} = [Ag^{+}][Cl^{-}]](/tpl/images/0619/3632/031c0.png)
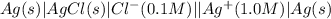
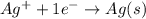 ,
, 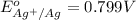
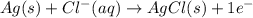 ,
, 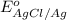 = ?
= ?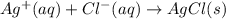
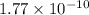
![E_{cell} = E^{o}_{cell} - \frac{0.0592 V}{n} log \frac{[AgCl]}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/d09c5.png)
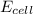 = 0.00 V
= 0.00 V![0.00 = E^{o}_{cell} - \frac{0.0592 V}{1} log \frac{1}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/1250d.png)