
Chemistry, 22.04.2020 22:08 tdahna0403
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product, phosgene (COCl2 ) CO(g) Cl2 (g) COCl2 (g) If the equilibrium constant for this reaction is Kc = 248, predict, if possible, what will happen when the reactants and product are combined with the concentrations shown. [CO] = [Cl2] = 0.010 M; [COCl2] = 0.070 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

You know the right answer?
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product,...
Questions



Biology, 29.08.2019 03:00



Biology, 29.08.2019 03:00



Mathematics, 29.08.2019 03:00

Mathematics, 29.08.2019 03:00

Geography, 29.08.2019 03:00


Mathematics, 29.08.2019 03:00


Arts, 29.08.2019 03:00

English, 29.08.2019 03:00

Chemistry, 29.08.2019 03:00

History, 29.08.2019 03:00

Mathematics, 29.08.2019 03:00

History, 29.08.2019 03:00

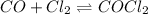
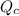 ) for this reaction is represented as:
) for this reaction is represented as:![Q_{c}=\frac{[COCl_{2}]}{[CO][Cl_{2}]}](/tpl/images/0619/3924/9faba.png)
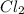 ] = 0.010 M and [
] = 0.010 M and [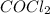 ] = 0.070 M
] = 0.070 M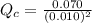 = 700
= 700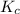 therefore reaction will proceed towards forward direction i.e. more
therefore reaction will proceed towards forward direction i.e. more

