
Chemistry, 22.04.2020 20:00 taralynnn8870
When a 0.031M aqueous solution of a certain acid is prepared, the acid is 0.89% dissociated. Calculate the acid dissociation constant Ka of the acid. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
When a 0.031M aqueous solution of a certain acid is prepared, the acid is 0.89% dissociated. Calcula...
Questions





English, 25.03.2021 03:00

Mathematics, 25.03.2021 03:00






Mathematics, 25.03.2021 03:00

Mathematics, 25.03.2021 03:00


Mathematics, 25.03.2021 03:00

English, 25.03.2021 03:00

Health, 25.03.2021 03:00

Mathematics, 25.03.2021 03:00

Mathematics, 25.03.2021 03:00

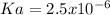
 due to the reaction extent, the percent dissociation is:
due to the reaction extent, the percent dissociation is:![\% Dissociation:\frac{x}{[acid]_0}](/tpl/images/0618/9112/63f66.png)
![x=\% Dissociation*[acid]_0=0.89\%*0.031M=2.759x10^{-4}M](/tpl/images/0618/9112/fb10b.png)
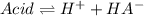
![Ka=\frac{x*x}{[acid]_0-x}=\frac{2.759x10^{-4}M*2.759x10^{-4}M}{0.031M-2.759x10^{-4}M} \\\\Ka=2.5x10^{-6}](/tpl/images/0618/9112/d4830.png)


