
The labels of bottles that contain barium hydroxide, hydrochloric acid and sodium carbonate had fallen off. When the contents of the bottle A were mixed with B's Contents, a white precipitate formed, A with C yielded a gas and B with C gave heat. Identify A, B,C and write molecular and net ionic equations for the chemical changes observed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
The labels of bottles that contain barium hydroxide, hydrochloric acid and sodium carbonate had fall...
Questions

Mathematics, 29.01.2021 02:10


Mathematics, 29.01.2021 02:10

Computers and Technology, 29.01.2021 02:10






World Languages, 29.01.2021 02:10

Spanish, 29.01.2021 02:10

Mathematics, 29.01.2021 02:10

Mathematics, 29.01.2021 02:10


Mathematics, 29.01.2021 02:10

Mathematics, 29.01.2021 02:10

Mathematics, 29.01.2021 02:10

Mathematics, 29.01.2021 02:10

Chemistry, 29.01.2021 02:10

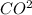 3(aq) +2H+(aq) -- C02(g) +H2O(l)
3(aq) +2H+(aq) -- C02(g) +H2O(l)


