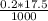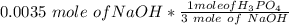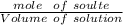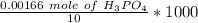Chemistry, 22.04.2020 15:42 hiitslillyhere
• We obtained the above 10.00-mL solution by diluting a stock solution using a 1.00-mL aliquot and placing it into a 25.00-mL volumetric flask and diluting to 25.00 mL prior to removing the 10.00 mL sample used above. What was the molar concentration of phosphoric acid in the original stock solution?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

You know the right answer?
• We obtained the above 10.00-mL solution by diluting a stock solution using a 1.00-mL aliquot and p...
Questions








Mathematics, 04.08.2020 14:01

History, 04.08.2020 14:01





Mathematics, 04.08.2020 14:01


Mathematics, 04.08.2020 14:01

Mathematics, 04.08.2020 14:01