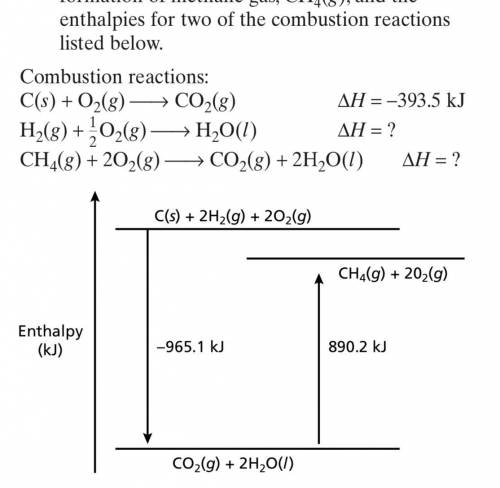
For certain molecules, enthalpies of formation
can be determined from combustion data. Using t...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2


Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Questions

Mathematics, 14.07.2020 19:01





Mathematics, 14.07.2020 19:01

History, 14.07.2020 19:01





Mathematics, 14.07.2020 19:01







Mathematics, 14.07.2020 19:01