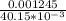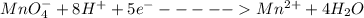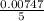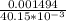Chemistry, 22.04.2020 04:39 aroland1990x
A Cu2+ solution is prepared by dissolving a 0.4749 g piece of copper wire in acid. The solution is then passed through a Walden reductor, reducing Cu2+ to Cu+ . The resulting Cu+ solution required 40.15 mL of each of the titrants to reach the endpoint. Calculate the concentration of each titrant.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
A Cu2+ solution is prepared by dissolving a 0.4749 g piece of copper wire in acid. The solution is t...
Questions

History, 14.01.2021 22:40


Biology, 14.01.2021 22:40

Mathematics, 14.01.2021 22:40



Mathematics, 14.01.2021 22:40


Mathematics, 14.01.2021 22:40

Mathematics, 14.01.2021 22:40

SAT, 14.01.2021 22:40


English, 14.01.2021 22:40




Mathematics, 14.01.2021 22:40

Mathematics, 14.01.2021 22:40

Chemistry, 14.01.2021 22:40

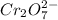 = 0.03101 M
= 0.03101 M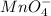 = 0.03721 M
= 0.03721 M
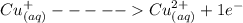
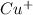 = 1 mole of
= 1 mole of 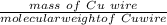
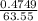
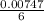
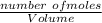
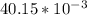 ; we have:
; we have: