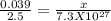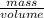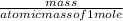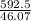Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
5. A penny weighs about 2.5 g. How many moles of pennies would be required to equal the mass of the...
Questions

Mathematics, 29.10.2020 19:20


Mathematics, 29.10.2020 19:20


Computers and Technology, 29.10.2020 19:20

Mathematics, 29.10.2020 19:20

Mathematics, 29.10.2020 19:20




Mathematics, 29.10.2020 19:20



Mathematics, 29.10.2020 19:20

History, 29.10.2020 19:20

Mathematics, 29.10.2020 19:20



English, 29.10.2020 19:20

Mathematics, 29.10.2020 19:20


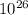 moles moles of pennies would be required to equal the mass of the moon.
moles moles of pennies would be required to equal the mass of the moon.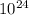 kg
kg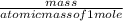
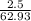
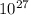 grams
grams