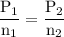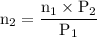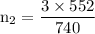Chemistry, 22.04.2020 03:59 davelopez979
A catalytic converter in an automobile uses a palladium or platinum catalyst to convert carbon monoxide gas to carbon dioxide according to the following reaction: 2CO(g) + O2 (g) --> 2CO2(g) A chemist researching the effectiveness of a new catalyst combines a 2.0 : 1.0 mole ratio mixture of carbon monoxide and oxygen gas (respectively) over the catalyst in a 2.45L flask at a total pressure of 740 torr and a temperature of 552 C . When the reaction is complete, the pressure in the flask has dropped to 552 torr. What percentage of the carbon monoxide was converted to carbon dioxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
A catalytic converter in an automobile uses a palladium or platinum catalyst to convert carbon monox...
Questions









Chemistry, 19.12.2019 21:31



Mathematics, 19.12.2019 21:31

History, 19.12.2019 21:31

Mathematics, 19.12.2019 21:31






 2CO₂
2CO₂