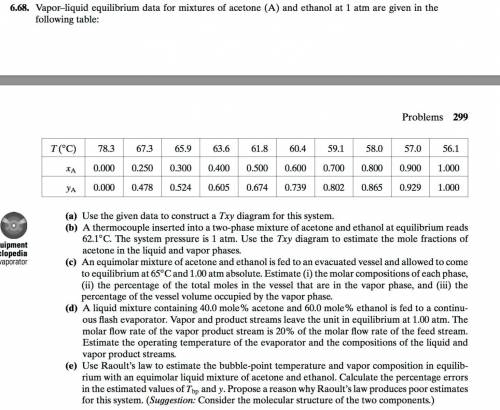
Chemistry, 22.04.2020 04:08 destiniout04231
An equimolar mixture of acetone and ethanol is fed to an evacuated vessel and allowedto come to equilibrium at 65°C and 1.00 atm absolute. State a quick way to show thatthe system has two phases. Estimate (i) the molar compositions of each phase, (ii) thepercentage of the total moles in the vessel that are in the vapor phase, and (iii) thepercentage of the vessel volume occupied by the vapor phase.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
An equimolar mixture of acetone and ethanol is fed to an evacuated vessel and allowedto come to equi...
Questions

Mathematics, 25.06.2019 10:20






Computers and Technology, 25.06.2019 10:20






Social Studies, 25.06.2019 10:20

Computers and Technology, 25.06.2019 10:20



English, 25.06.2019 10:20

Mathematics, 25.06.2019 10:20

Physics, 25.06.2019 10:20

Mathematics, 25.06.2019 10:20