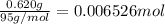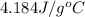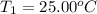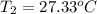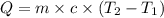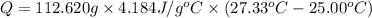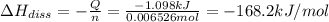Chemistry, 22.04.2020 03:29 milkshakegrande101
The salt magnesium chloride is soluble in water. When 0.620 g MgCl2 is dissolved in 112.00 g water, the temperature of the solution increases from 25.00 °C to 27.33 °C. Based on this observation, calculate the dissolution enthalpy, ΔdissH, of MgCl2. Assume that the specific heat capacity of the solution is 4.184 J g-1 °C-1 and that the energy transfer to the calorimeter is negligible. ΔdissH = kJ/mol

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
The salt magnesium chloride is soluble in water. When 0.620 g MgCl2 is dissolved in 112.00 g water,...
Questions



Computers and Technology, 30.08.2019 00:30

Medicine, 30.08.2019 00:30



Chemistry, 30.08.2019 00:30


Mathematics, 30.08.2019 00:30

Business, 30.08.2019 00:30



Computers and Technology, 30.08.2019 00:30


History, 30.08.2019 00:30

History, 30.08.2019 00:30

Biology, 30.08.2019 00:30

Computers and Technology, 30.08.2019 00:30

English, 30.08.2019 00:30