
Chemistry, 22.04.2020 02:08 alexis9263
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b) the entropy of the solution must be greater than that of its pure components. c) H bonds must exist between solvent and solute. d) the entropy of the solution is immaterial. e) strong ion-dipole forces must exist in the solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b)...
Questions

English, 19.10.2021 06:10













History, 19.10.2021 06:20



Mathematics, 19.10.2021 06:20



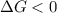 .
.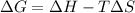 .......... (1)
.......... (1)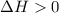 (for an endothermic process).
(for an endothermic process).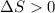 ) more will be the negative value of
) more will be the negative value of 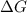 .
.

