
Chemistry, 22.04.2020 01:56 julielebo8
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction system. b. the increased molecular disorder that occurs in the system. c. the energy given off to the surroundings. d. the direction in which a net reaction occurs. e. the excess entropy given off to the surroundings

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1


Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction...
Questions

English, 11.03.2021 14:50


Mathematics, 11.03.2021 14:50


Mathematics, 11.03.2021 14:50

English, 11.03.2021 14:50


Mathematics, 11.03.2021 14:50

Geography, 11.03.2021 14:50



English, 11.03.2021 15:00

Mathematics, 11.03.2021 15:00







Mathematics, 11.03.2021 15:00

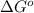 . Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.
. Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.

