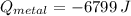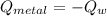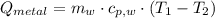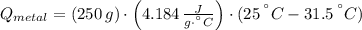Chemistry, 22.04.2020 02:39 rodolfoperezzz1332
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initially at 25.0 oC. The final temperature of the water is 31.5 oC. What is the heat change of the metal in joules? The specific heat of water is 4.184 J/goC

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initial...
Questions



Mathematics, 18.10.2020 08:01



Mathematics, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01


Law, 18.10.2020 08:01



Mathematics, 18.10.2020 08:01


Mathematics, 18.10.2020 08:01


Chemistry, 18.10.2020 08:01