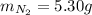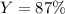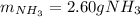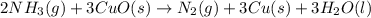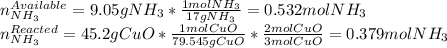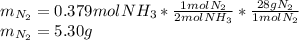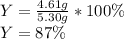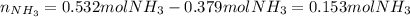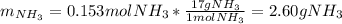Chemistry, 22.04.2020 01:43 sayedabdullah
2 NH3(g) + 3 CuO(s) → N2(g) + 3 Cu(s) + 3 H2O(l) a. What is the limiting reagent when 9.05 g of NH3 reacted with 45.2 g of CuO?(5 points) b. How many grams of N2 can be made?(10 points) c. If 4.61 g of N2 are made, what is the percent yield? (5 points) d. What is the mass of the excess reactant that remains after the reaction. (10 points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
2 NH3(g) + 3 CuO(s) → N2(g) + 3 Cu(s) + 3 H2O(l) a. What is the limiting reagent when 9.05 g of NH3...
Questions




Mathematics, 06.11.2019 21:31


English, 06.11.2019 21:31







Computers and Technology, 06.11.2019 21:31