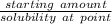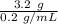Chemistry, 22.04.2020 01:02 SKYBLUE1015
Compound A has a solubility of 0.2 g/mL in toluene at toluene's boiling point and a solubility of 0.05 g/mL at 0 ºC. How much toluene would be necessary to recrystallize 3.2 g of A. What would be the maximum amount of A that could be recovered if the saturated solution was allowed to cool to 0 ºC. How much A would be recovered, if you accidentally used twice as much toluene as was necessary?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
Compound A has a solubility of 0.2 g/mL in toluene at toluene's boiling point and a solubility of 0....
Questions

History, 14.04.2020 21:06

Spanish, 14.04.2020 21:06







Arts, 14.04.2020 21:06








Mathematics, 14.04.2020 21:06


Mathematics, 14.04.2020 21:06

Mathematics, 14.04.2020 21:06