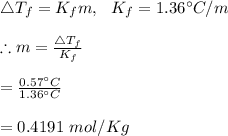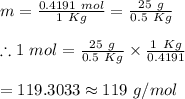Chemistry, 22.04.2020 01:46 slowik9467
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
0.57 °C what is the molecular weight of the compound assume no molecular disassociation upon
dissolution Kf equals 1.36 °C/m
O 119 g/mol
90 g/mol
0 60 g/mol
238 g/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
Questions


Arts, 03.07.2019 05:00

History, 03.07.2019 05:00


Chemistry, 03.07.2019 05:00



Computers and Technology, 03.07.2019 05:00

Mathematics, 03.07.2019 05:00

Chemistry, 03.07.2019 05:00

Business, 03.07.2019 05:00

History, 03.07.2019 05:00

English, 03.07.2019 05:00


Mathematics, 03.07.2019 05:00

Mathematics, 03.07.2019 05:00




Spanish, 03.07.2019 05:00