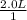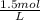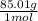Chemistry, 22.04.2020 00:20 venuscagle
1. What mass of the following chemicals is needed to make the solutions indicated?
a. 1.0 liter of a 1.0 M mercury (II) chloride (HgCl2) solution
b. 2.0 liters of a 1.5 M sodium nitrate (NaNO3) solution
c. 5.0 liters of a 0.1 M Ca(OH)2 solution
d. 100 mL of a 0.5 M (NH4)3PO4 solution
2. Calculate the molarity of the following solutions.
a. 12 g of lithium hydroxide (LiOH) in 1.0 L of solution
b. 198 g of barium bromide (BaBr2) in 2.0 L of solution
c. 54 g of calcium sulfide (CaS) in 3.0 L of solution
3. Calculate the volume of each solution, in liters.
a. a 1.0 M solution containing 85 g of silver nitrate (AgNO3)
b. a 0.5 M solution containing 250 g of manganese (II) chloride (MnCl2)
c. a 0.4 M solution containing 290 g of aluminum nitrate (Al(NO3)3)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1


Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
1. What mass of the following chemicals is needed to make the solutions indicated?
a. 1....
a. 1....
Questions

Social Studies, 19.08.2021 21:00



History, 19.08.2021 21:00



Mathematics, 19.08.2021 21:00








Mathematics, 19.08.2021 21:00


History, 19.08.2021 21:00

Mathematics, 19.08.2021 21:00

Health, 19.08.2021 21:00

English, 19.08.2021 21:00