
Chemistry, 21.04.2020 23:23 jordynj6363
Question 16 A chemistry graduate student is given of a methylamine solution. Methylamine is a weak base with . What mass of should the student dissolve in the solution to turn it into a buffer with pH ? You may assume that the volume of the solution doesn't change when the is dissolved in it. Be sure your answer has a unit symbol, and round it to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Question 16 A chemistry graduate student is given of a methylamine solution. Methylamine is a weak b...
Questions

Mathematics, 16.02.2020 04:47

Advanced Placement (AP), 16.02.2020 04:47



Chemistry, 16.02.2020 04:48

History, 16.02.2020 04:48

Mathematics, 16.02.2020 04:48


Social Studies, 16.02.2020 04:48

Biology, 16.02.2020 04:48

English, 16.02.2020 04:49

Mathematics, 16.02.2020 04:49



Mathematics, 16.02.2020 05:01

Computers and Technology, 16.02.2020 05:01

Mathematics, 16.02.2020 05:01



History, 16.02.2020 05:02

![\frac{[Methylamine]}{[Salt]}](/tpl/images/0616/2055/75c38.png)
![\frac{1.70}{[Salt]}](/tpl/images/0616/2055/8434c.png) -0.24 = log
-0.24 = log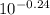 =
=

