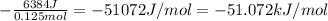Chemistry, 21.04.2020 22:31 cassidy100117
When 4.98 g of NaOH was dissolved in 52.79 g of water in a calorimeter at 23.7 oC, the temperature of the solution went up to 50.1 oC. What is the enthalpy change in kJ/mole of sodium hydroxide? Assume the specific heat of the mixture is the same as water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1


Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
When 4.98 g of NaOH was dissolved in 52.79 g of water in a calorimeter at 23.7 oC, the temperature o...
Questions












English, 30.10.2020 17:40


English, 30.10.2020 17:40



Health, 30.10.2020 17:40

Spanish, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40

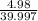 moles of NaOH = 0.125 moles of NaOH
moles of NaOH = 0.125 moles of NaOH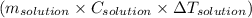 , where m is mass C is specific heat and
, where m is mass C is specific heat and  is change in temperature.
is change in temperature.![[57.77g\times 4.186\frac{J}{g.^{0}\textrm{C}}\times (50.1-23.7)^{0}\textrm{C}]](/tpl/images/0616/0525/cdabc.png)