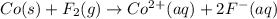Assign oxidation states to all of the species in the following redox reaction. For the reactants, identify electron loss or gain, the species oxidized, the species reduced, the oxidizing agent and the reducing agent. Co(s) + F2(g) Co2+(aq) + 2F-(aq) Oxidation state Electron loss or gain Oxidized or reduced Reducing or oxidizing agent

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Assign oxidation states to all of the species in the following redox reaction. For the reactants, id...
Questions


History, 14.12.2020 22:00



Mathematics, 14.12.2020 22:00



Mathematics, 14.12.2020 22:00

Mathematics, 14.12.2020 22:00


Mathematics, 14.12.2020 22:00


English, 14.12.2020 22:00

Mathematics, 14.12.2020 22:00



Advanced Placement (AP), 14.12.2020 22:00



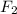 gain two electrons and thus gets reduced and acts as oxidizing agent.
gain two electrons and thus gets reduced and acts as oxidizing agent.