
Chemistry, 21.04.2020 21:47 sustaitaj2022
The reaction was studied at a series of different temperatures. A plot of ln(k) vs. 1/T gave a straight line relationship with a slope of -693 and a y-intercept of -0.425. Additionally, a study of the concentration of A with respect to time showed that only a plot of ln[A] vs. time gave a straight line relationship. What is the initial rate of this reaction when [A] = 0.41 at 271 K?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1


Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
The reaction was studied at a series of different temperatures. A plot of ln(k) vs. 1/T gave a strai...
Questions

Social Studies, 18.08.2019 23:50


Mathematics, 18.08.2019 23:50

Mathematics, 18.08.2019 23:50



Business, 18.08.2019 23:50

Social Studies, 18.08.2019 23:50


Geography, 18.08.2019 23:50

Mathematics, 18.08.2019 23:50


Mathematics, 18.08.2019 23:50



English, 18.08.2019 23:50

Mathematics, 18.08.2019 23:50

English, 18.08.2019 23:50

Social Studies, 18.08.2019 23:50

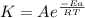
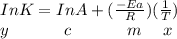
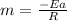
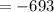
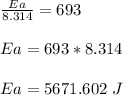
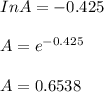
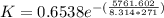
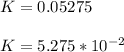
![\frac{1}{[A]}](/tpl/images/0615/8431/153b1.png) vs time is a straight line relationship;
vs time is a straight line relationship;

