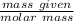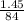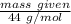Chemistry, 21.04.2020 19:26 youngchapo813p8d9u1
3. Calculate the theoretical value for the number of moles of CO2 that should have been produced in each balloon assuming that 1.45 g of NaHCO3 is present in an antacid tablet. Use stoichiometry (a mole ratio conversion must be present) to find your answers (there should be three: one answer for each balloon). (6 pts)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
3. Calculate the theoretical value for the number of moles of CO2 that should have been produced in...
Questions


Mathematics, 18.01.2021 22:50






History, 18.01.2021 22:50


Spanish, 18.01.2021 22:50


Health, 18.01.2021 22:50



Mathematics, 18.01.2021 22:50

Arts, 18.01.2021 22:50

Health, 18.01.2021 22:50



Advanced Placement (AP), 18.01.2021 22:50

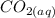 is 0.0173 moles
is 0.0173 moles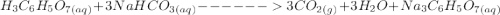
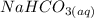 reacts with
reacts with 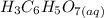 to produce 3 moles of
to produce 3 moles of