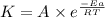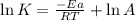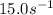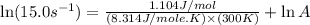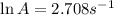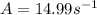A chemical reaction has an energy of activation Ea = 1∙104 J mol-1 at T = 300 K. The first-order rate constant for this reaction was found to be 15.0 s-1. In the presence of a catalyst, the activation energy is reduced to 1∙103 J mol-1. Calculate the pre-exponential factor in the Arrhenius equation

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
What type of scientific model does the flow chart represent? (a chart of the scientific process) a. conceptual b. mathematical c. physical d. virtual
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
A chemical reaction has an energy of activation Ea = 1∙104 J mol-1 at T = 300 K. The first-order rat...
Questions


English, 22.03.2021 22:00




Mathematics, 22.03.2021 22:00

History, 22.03.2021 22:00








History, 22.03.2021 22:00

English, 22.03.2021 22:00

Mathematics, 22.03.2021 22:00


Mathematics, 22.03.2021 22:00