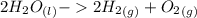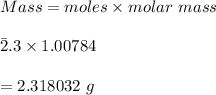Chemistry, 21.04.2020 18:47 PONBallfordM89
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseous elements. How many grams of hydrogen gas should get (theoretical yield)? The chemist collected 2.0 moles hydrogen gas. What is his percent yield?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseo...
Questions




Social Studies, 18.12.2020 06:30


Mathematics, 18.12.2020 06:30





Advanced Placement (AP), 18.12.2020 06:30

Mathematics, 18.12.2020 06:30

History, 18.12.2020 06:30

History, 18.12.2020 06:30





Biology, 18.12.2020 06:30