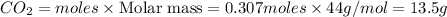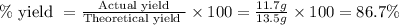Chemistry, 21.04.2020 18:45 hardwick744
When limestone rock, which is principally calcium carbonate, is heated, a reaction occurs. If 11.7 g of carbon dioxide were produced in the lab from the decomposition of 30.7g of calcium carbonate, what is the percent yield for the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
When limestone rock, which is principally calcium carbonate, is heated, a reaction occurs. If 11.7 g...
Questions





Mathematics, 10.07.2019 00:50


Mathematics, 10.07.2019 00:50

Mathematics, 10.07.2019 00:50


Mathematics, 10.07.2019 00:50

Mathematics, 10.07.2019 00:50

English, 10.07.2019 00:50

English, 10.07.2019 00:50

Mathematics, 10.07.2019 00:50

Mathematics, 10.07.2019 00:50

English, 10.07.2019 00:50

Mathematics, 10.07.2019 00:50



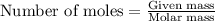
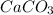
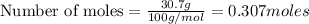

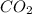
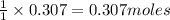 of
of