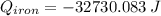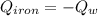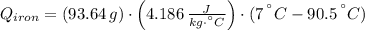Chemistry, 21.04.2020 17:37 kaylaanderson348
A piece of iron with a mass of 56.2 grams is heated and placed into a calorimeter containing 93.64 grams of water at 7.0 degrees Celsius. The final temperature of the water and the iron is 90.5 degrees Celsius. Assuming no heat is lost to the surroundings, how much heat (in Joules) does the iron release? Round your answer to the nearest 0.1 Joules.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
A piece of iron with a mass of 56.2 grams is heated and placed into a calorimeter containing 93.64 g...
Questions

Mathematics, 22.03.2021 16:20

Chemistry, 22.03.2021 16:20


Mathematics, 22.03.2021 16:20

English, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20


Mathematics, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

Social Studies, 22.03.2021 16:20

Computers and Technology, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20


Biology, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20

English, 22.03.2021 16:20


Computers and Technology, 22.03.2021 16:20

Mathematics, 22.03.2021 16:20