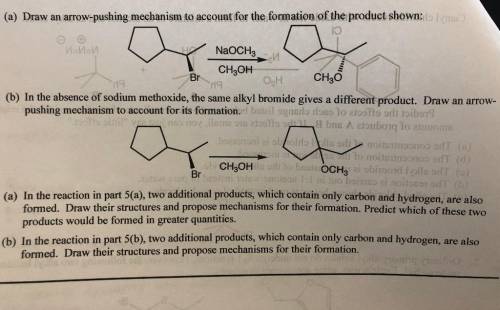
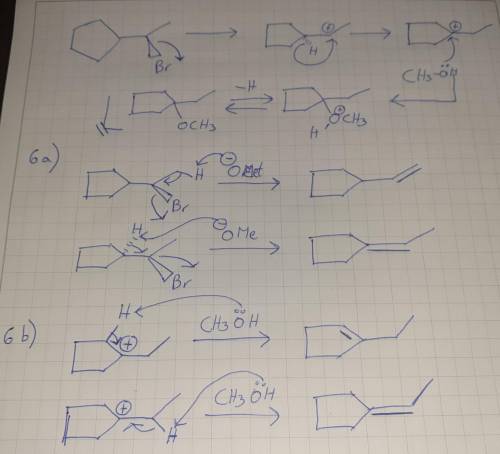
Chemistry, 21.04.2020 16:56 bankrollbaby01
In the absence of sodium methoxide, the same alkyl bromide gives a different product. Draw an arrowpushing mechanism to account for its formation. 6. (a) In the reaction in part 5(a), two additional products, which contain only carbon and hydrogen, are also formed. Draw their structures and propose mechanisms for their formation. Predict which of these two products would be formed in greater quantities. (b) In the reaction in part 5(b), two additional products, which contain only carbon

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3
You know the right answer?
In the absence of sodium methoxide, the same alkyl bromide gives a different product. Draw an arrowp...
Questions

Mathematics, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40



Mathematics, 23.02.2021 02:40

English, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40



History, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40




Mathematics, 23.02.2021 02:40



Mathematics, 23.02.2021 02:40