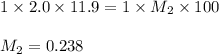Chemistry, 21.04.2020 16:29 elirosejohlandancel
An old bottle labeled Standardized 5.0 M NaOH was found at the back of a shelf in the stockroom. To determine whether the concentration was still 5.0 M, 5.0 mL of the solution was diluted to 100 mL and titrated to the equivalence point with 11.9 mL of 2.0 M HCl(aq). What is the molarity of the sodium hydroxide solution in the bottle

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
An old bottle labeled Standardized 5.0 M NaOH was found at the back of a shelf in the stockroom. To...
Questions



Mathematics, 14.02.2020 15:37

Geography, 14.02.2020 15:37

History, 14.02.2020 15:38

English, 14.02.2020 15:38







English, 14.02.2020 16:04

Mathematics, 14.02.2020 16:05


English, 14.02.2020 16:05


Computers and Technology, 14.02.2020 16:05


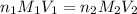
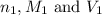 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 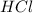
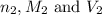 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.