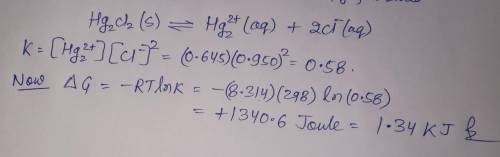Chemistry, 21.04.2020 04:51 micahwilkerson9495
A chemist fills a reaction vessel with 0.623g mercurous chloride(Hg2Cl2) solid, 0.645M mercury (I) (Hg2^2+)aqueous solution, and 0.905M chloride (Cl-) aqueous solution at a temperature of 25.0°C.
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction:
Hg2Cl2(s) ⇌ Hg2^2+ (aq) + 2Cl^- (aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
A chemist fills a reaction vessel with 0.623g mercurous chloride(Hg2Cl2) solid, 0.645M mercury (I) (...
Questions

World Languages, 30.10.2019 16:31


Mathematics, 30.10.2019 16:31

Mathematics, 30.10.2019 16:31

Mathematics, 30.10.2019 16:31



Mathematics, 30.10.2019 16:31



Computers and Technology, 30.10.2019 16:31

History, 30.10.2019 16:31

Biology, 30.10.2019 16:31



Mathematics, 30.10.2019 16:31


Biology, 30.10.2019 16:31

Mathematics, 30.10.2019 16:31

Mathematics, 30.10.2019 16:31