Consider the reaction:
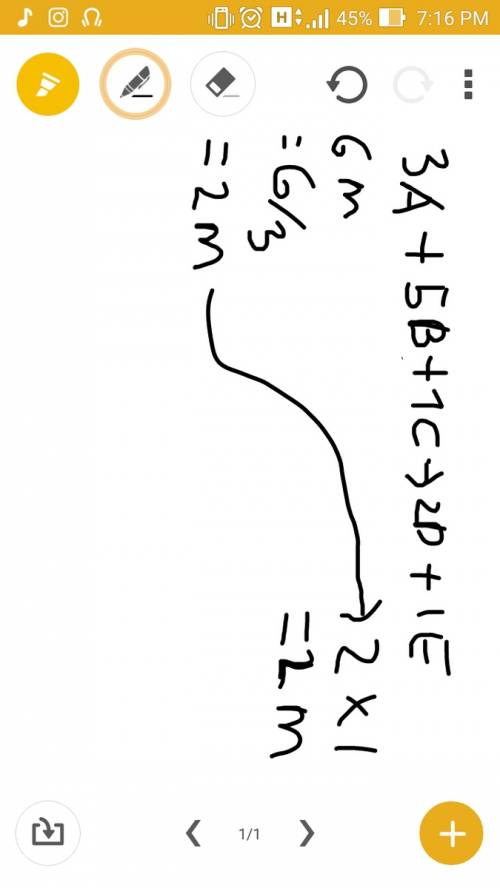
3a +5b + 1c --> 2d + 1e
if you have 6 moles of reactant a an...

Chemistry, 29.08.2019 01:30 zawnghkawng64361
Consider the reaction:
3a +5b + 1c --> 2d + 1e
if you have 6 moles of reactant a and excess of b and c, how much product e would be formed? show work so i can understand .

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
Questions

Mathematics, 12.01.2021 22:10






Mathematics, 12.01.2021 22:10

Arts, 12.01.2021 22:10




Mathematics, 12.01.2021 22:10


Chemistry, 12.01.2021 22:10

Mathematics, 12.01.2021 22:10

Mathematics, 12.01.2021 22:10


Mathematics, 12.01.2021 22:10

History, 12.01.2021 22:10

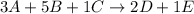
 moles of 'E'
moles of 'E'

