Chemistry, 21.04.2020 04:12 Riplilpeep
A gas occupies 3.5 L at standard pressure. Find the volume of the gas when the pressure is 1.5 atm. (Remember, standard pressure equals 101.3kPa, 1atm, or 760mmHg.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
A gas occupies 3.5 L at standard pressure. Find the volume of the gas when the pressure is 1.5 atm....
Questions


Mathematics, 29.03.2021 22:10








Mathematics, 29.03.2021 22:10

Biology, 29.03.2021 22:10



Mathematics, 29.03.2021 22:10




Mathematics, 29.03.2021 22:10


Spanish, 29.03.2021 22:10

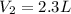
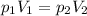
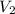 , we obtain:
, we obtain: