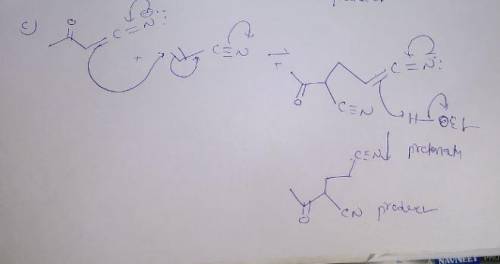
The Michael reaction is a conjugate addition process wherein a nucleophilic enolate anion (the donor) reacts with an α,β-unsaturated carbonyl compound (the acceptor). The best Michael reactions are those that take place when a particularly stable enolate anion is formed via treatment of the donor with a strong base. Alternatively, milder conditions can be used if an enamine is chosen as the donor, this variant is termed the Stork reaction. In the second step, the donor adds to the β-carbon of the acceptor in a conjugate addition, generating a new enolate. The enolate abstracts a proton from solvent or from a new donor molecule to give the conjugate addition product.
Draw curved arrows to show the movement of electrons in this step of the mechanism.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
The Michael reaction is a conjugate addition process wherein a nucleophilic enolate anion (the donor...
Questions

Mathematics, 11.05.2021 02:00





Chemistry, 11.05.2021 02:00

English, 11.05.2021 02:00







Arts, 11.05.2021 02:00

Mathematics, 11.05.2021 02:00


Mathematics, 11.05.2021 02:00



Mathematics, 11.05.2021 02:00