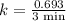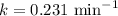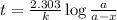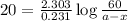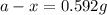Chemistry, 21.04.2020 00:37 alyssamiller401
An important application of exponential functions is working with half-life of radioactive isotopes in chemistry. These isotopes emit particles and decay into stable forms, in doing so they lose mass over time. Half-life of an isotope is the time it takes for the amount to decay by half. For example, the half life of Bromine-85 is 3 minutes. This means if you start with 60g of Br-85, 3 minutes later 30g will remain. How much Br-85 will remain after 20 minutes?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3
You know the right answer?
An important application of exponential functions is working with half-life of radioactive isotopes...
Questions


Mathematics, 29.05.2020 05:01

English, 29.05.2020 05:01




History, 29.05.2020 05:01










Chemistry, 29.05.2020 05:01



Social Studies, 29.05.2020 05:01