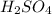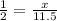Chemistry, 21.04.2020 00:37 hailey5129
Sulfuric acid is formed when sulfur dioxide reacts with oxygen and water. Write a balanced chemical equation for the reaction. (Use the lowest possible coefficients. Include states of matter under the SATP conditions in your answer.)
How many mol H2SO4 is produced from 11.5 moles of SO2?
How many O2 is needed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2


Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
Sulfuric acid is formed when sulfur dioxide reacts with oxygen and water. Write a balanced chemical...
Questions

Mathematics, 14.12.2020 21:10


History, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Biology, 14.12.2020 21:10









Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10



Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

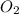 (g) +
(g) + 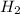 O (l) ⇒
O (l) ⇒