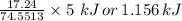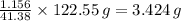Chemistry, 20.04.2020 22:22 albattatasraap5wymy
Dissolving potassium chlorate (KClO3) is even more endothermic than potassium chloride.
Your task is to determine how many grams of potassium chlorate you would have to add to 100 mL of water to produce the same temperature change as 5 grams of KCl.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Dissolving potassium chlorate (KClO3) is even more endothermic than potassium chloride.
Your...
Your...
Questions

Mathematics, 21.01.2020 23:31

Geography, 21.01.2020 23:31


English, 21.01.2020 23:31


History, 21.01.2020 23:31


Mathematics, 21.01.2020 23:31




History, 21.01.2020 23:31


Spanish, 21.01.2020 23:31

History, 21.01.2020 23:31


History, 21.01.2020 23:31

History, 21.01.2020 23:31

Mathematics, 21.01.2020 23:31