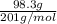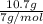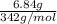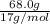Chemistry, 30.09.2019 06:30 genyjoannerubiera
Determine the number of atoms in each sample.
a.) 98.3 g mercury, hk
c.) 10.7 g lithium, li
determine the number of moles in each sample.
a.) 6.84g sucrose c12h22)11
c.) 68.0g ammonia, nh3
determine the mass of the following molar quantity.
a.) 3.52 mol si
c.) 0.550 mol f2
chemistry, year1, honors. can *someone* me?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Determine the number of atoms in each sample.
a.) 98.3 g mercury, hk
c.) 1...
a.) 98.3 g mercury, hk
c.) 1...
Questions

Mathematics, 02.02.2020 09:42


Mathematics, 02.02.2020 09:42




Mathematics, 02.02.2020 09:42


Social Studies, 02.02.2020 09:42

Social Studies, 02.02.2020 09:42


Chemistry, 02.02.2020 09:42

Health, 02.02.2020 09:42


Health, 02.02.2020 09:42




Biology, 02.02.2020 09:43

Mathematics, 02.02.2020 09:43