
Chemistry, 20.04.2020 16:55 zeesharpe05
How many liters of hydrogen gas will be produced at STP from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (H3PO4)
The equation is 3Mg + 2H3(PO4)-->Mg(PO4)2+3H2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
How many liters of hydrogen gas will be produced at STP from the reaction of 7.179×10^23 atoms of ma...
Questions

History, 14.06.2021 08:30






Mathematics, 14.06.2021 08:30

Mathematics, 14.06.2021 08:30

Computers and Technology, 14.06.2021 08:30


Chemistry, 14.06.2021 08:30

Chemistry, 14.06.2021 08:40



Spanish, 14.06.2021 08:40

Mathematics, 14.06.2021 08:40


Mathematics, 14.06.2021 08:40

Chemistry, 14.06.2021 08:40

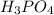 = 54.219 g
= 54.219 g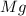 =
= 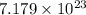
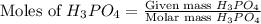
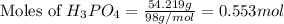
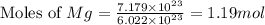
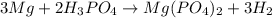
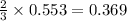 moles of
moles of 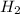
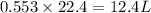 volume of hydrogen gas
volume of hydrogen gas

