
Chemistry, 20.04.2020 14:30 jflandersneongr
Aluminum Oxide is formed when aluminum combines with oxygen in the air. How many grams of Al2O3 are formed when 23.6g of Al reacts completely with oxygen?
4 Al + 3 O2 —> 2 Al2O3
A. 44.6 grams B. 35.6 grams C. 87.6 grams D. 21.6 grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Aluminum Oxide is formed when aluminum combines with oxygen in the air. How many grams of Al2O3 are...
Questions

Mathematics, 19.08.2019 08:30

Geography, 19.08.2019 08:30


Chemistry, 19.08.2019 08:30

Physics, 19.08.2019 08:30


History, 19.08.2019 08:30

Mathematics, 19.08.2019 08:30


Biology, 19.08.2019 08:30

Mathematics, 19.08.2019 08:30


Mathematics, 19.08.2019 08:30


Mathematics, 19.08.2019 08:30


Mathematics, 19.08.2019 08:30

English, 19.08.2019 08:30


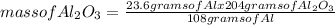
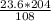 = 44.6 g of Al₂O₃
= 44.6 g of Al₂O₃ 

