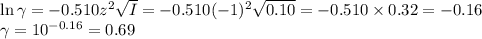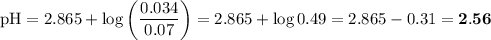We mix 0.08 moles of chloroacetic acid (ClCH2COOH) and 0.04 moles of
sodium chloroacetate (ClCH2COONa) in 1.0 L of water (pKa = 2,865).
to. Calculate the pH
yes. Calculate the pH using the formal forms (activities). Have on
counts the contribution of the protons (section a) in the calculation of the ionic strength.
C. Find the pH of a mixture prepared by dissolving the following compounds
in a final volume of 1L: 0.08 moles of ClCH2COOH, 0.04 moles of
ClCH2COONa, 0.05 moles of HNO3 and 0.06 moles of NaOH

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2



Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
We mix 0.08 moles of chloroacetic acid (ClCH2COOH) and 0.04 moles of
sodium chloroacetate (ClC...
sodium chloroacetate (ClC...
Questions

Mathematics, 12.09.2019 03:30



Geography, 12.09.2019 03:30


Mathematics, 12.09.2019 03:30





Mathematics, 12.09.2019 03:30

Mathematics, 12.09.2019 03:30


Mathematics, 12.09.2019 03:30

Mathematics, 12.09.2019 03:30

Mathematics, 12.09.2019 03:30



Mathematics, 12.09.2019 03:30

Mathematics, 12.09.2019 03:30

![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 2.865 +\log \left(\dfrac{0.04}{0.08}\right )\\\\& = & 2.865 + \log0.50 \\& = &2.865 - 0.30 \\& = & \mathbf{2.56}\\\end{array}](/tpl/images/0610/7910/ebc2c.png)
![\text{[H$^{+}$]} = 10^{-\text{pH}} \text{ mol/L} = 10^{-2.56}\text{ mol/L} = 2.73 \times 10^{-3}\text{ mol/L}](/tpl/images/0610/7910/adeed.png)
![I = \dfrac{1}{2} \sum_{i} {c_{i}z_{i}^{2}}\\\\I = \dfrac{1}{2}\left [0.04\times (+1)^{2} + 0.04\times(-1)^{2} + 0.00273\times(+1)^{2}\right]\\\\= \dfrac{1}{2} (0.04 + 0.04 + 0.00273) = \dfrac{1}{2} \times 0.08273 = 0.041](/tpl/images/0610/7910/9dfcb.png)
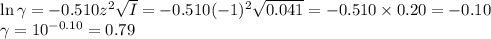
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{a_{\text{A}^{-}}}{a_{\text{[HA]}}}\right )\\\\& = & 2.865 +\log \left(\dfrac{0.032}{0.08}\right )\\\\& = & 2.865 + \log0.40 \\& = & 2.865 -0.40\\& = & \mathbf{2.46}\\\end{array}\\](/tpl/images/0610/7910/7d363.png)
![I = \dfrac{1}{2}\left [0.10\times (+1)^{2} + 0.05 \times(-1)^{2} + 0.05\times(-1)^{2}\right]\\\\= \dfrac{1}{2} (0.10 + 0.05 + 0.05) = \dfrac{1}{2} \times 0.20 = 0.10](/tpl/images/0610/7910/85fb3.png)