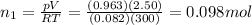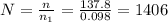Chemistry, 17.04.2020 22:16 gizmo50245
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 ∞C. The He is used to fill balloons to a volume of 2.50 L at 732 mm Hg and 27 ∞C. How many balloons can be filled with He? Assume that the cylinder can provide He until its internal pressure reaches 1.00 atm (i. e., there are 131 atmospheres of usable He in the cylinder).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 ∞C. The He is...
Questions



History, 01.12.2019 23:31

Social Studies, 01.12.2019 23:31

Social Studies, 01.12.2019 23:31


English, 01.12.2019 23:31

Mathematics, 01.12.2019 23:31

Chemistry, 01.12.2019 23:31

English, 01.12.2019 23:31

Mathematics, 01.12.2019 23:31

Mathematics, 01.12.2019 23:31


Social Studies, 01.12.2019 23:31


Business, 01.12.2019 23:31

Mathematics, 01.12.2019 23:31

Advanced Placement (AP), 01.12.2019 23:31


History, 01.12.2019 23:31

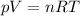
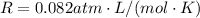 is the gas constant
is the gas constant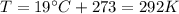 is the temperature
is the temperature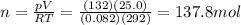
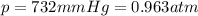 is the pressure
is the pressure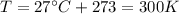 is the temperature
is the temperature