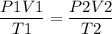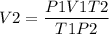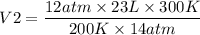Il initially have à gas at a pressure of 12 atm, a volume of 23 liters, and a
temperature of 2...

Chemistry, 17.04.2020 09:38 GamerGirl15
Il initially have à gas at a pressure of 12 atm, a volume of 23 liters, and a
temperature of 200 K, and then I raise the pressure to 14 atm and
increase the temperature to 300 K, what is the new volume of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

You know the right answer?
Questions

Mathematics, 27.08.2019 10:30

History, 27.08.2019 10:30




Mathematics, 27.08.2019 10:30

Social Studies, 27.08.2019 10:30


Biology, 27.08.2019 10:30

History, 27.08.2019 10:30

History, 27.08.2019 10:30


English, 27.08.2019 10:30

Biology, 27.08.2019 10:30


Mathematics, 27.08.2019 10:30


Mathematics, 27.08.2019 10:30