
Chemistry, 17.04.2020 05:06 shaunarothh1276
What volume of H2O(g) is produced when 3.30mol of C2H2(g) reacts with oxygen gas at STP?
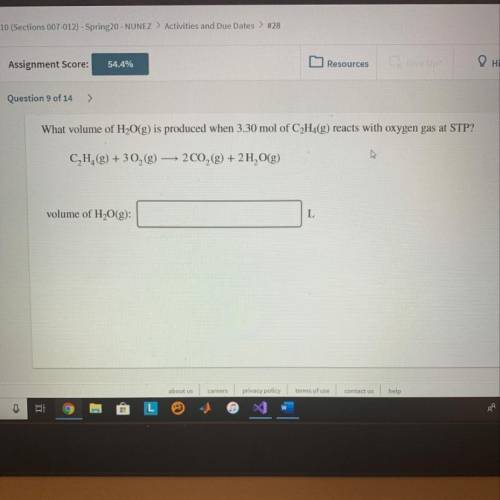

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

You know the right answer?
What volume of H2O(g) is produced when 3.30mol of C2H2(g) reacts with oxygen gas at STP?
...
...
Questions


Mathematics, 27.03.2020 19:06


Engineering, 27.03.2020 19:06




English, 27.03.2020 19:07


Mathematics, 27.03.2020 19:07

History, 27.03.2020 19:07

Mathematics, 27.03.2020 19:07

Mathematics, 27.03.2020 19:07




Mathematics, 27.03.2020 19:07


Mathematics, 27.03.2020 19:07

Arts, 27.03.2020 19:07




