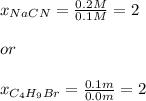Chemistry, 16.04.2020 23:33 harodkdc7910
If the rate of reaction of [0.1 M] sodium cyanide with [0.1 M] 2-bromo-2-methylpropane is 1.2 x 10-3 M/s, what would be the effect on the overall rate if the concentration of sodium cyanide is increased to [0.2 M] and the concentration of the alkyl bromide is decreased to [0.05 M]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
If the rate of reaction of [0.1 M] sodium cyanide with [0.1 M] 2-bromo-2-methylpropane is 1.2 x 10-3...
Questions

Chemistry, 26.09.2021 22:10

Mathematics, 26.09.2021 22:10

Computers and Technology, 26.09.2021 22:10


English, 26.09.2021 22:10

Physics, 26.09.2021 22:10

English, 26.09.2021 22:10


Business, 26.09.2021 22:10

English, 26.09.2021 22:10

Mathematics, 26.09.2021 22:10

Biology, 26.09.2021 22:10

Mathematics, 26.09.2021 22:10

Health, 26.09.2021 22:10


Mathematics, 26.09.2021 22:10

Mathematics, 26.09.2021 22:10

Chemistry, 26.09.2021 22:10


Mathematics, 26.09.2021 22:10