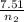A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
th...

Chemistry, 16.04.2020 23:03 Arianaagosto
A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
then added to the container until it reaches a final volume of 13.9 L. Assuming the
pressure and the temperature of the gas remained constant, calculate the moles of gas
added to the container.
15.1 mol
O 5.5 mol
0 4.40 mol
12.8 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Questions


Arts, 14.12.2020 19:00



Mathematics, 14.12.2020 19:00



Mathematics, 14.12.2020 19:00

History, 14.12.2020 19:00


Mathematics, 14.12.2020 19:00

Mathematics, 14.12.2020 19:00


Geography, 14.12.2020 19:00


Mathematics, 14.12.2020 19:00


Mathematics, 14.12.2020 19:00


Mathematics, 14.12.2020 19:00

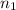 ) = 7.51 moles
) = 7.51 moles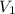 ) = 8.15 L
) = 8.15 L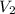 ) = 13.9 L
) = 13.9 L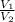 =
= 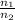
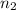 represents the final moles
represents the final moles 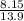 =
=