Chemistry, 16.04.2020 22:09 zenaidazurita1p6bs1d
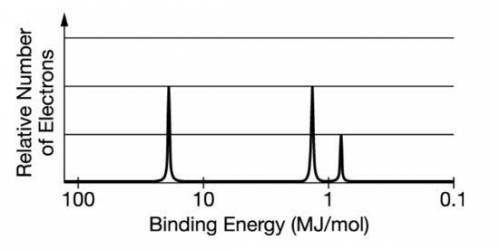
The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom? The spectrum shows an odd number electrons. A The spectrum shows a single electron in the 2p subshell. B The spectrum shows equal numbers of electrons in the first and second electron shells. C The spectrum shows three electrons with the same binding energy in the second electron shell.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
The photoelectron spectrum for the element boron is represented above. Which of the following best e...
Questions


Mathematics, 31.10.2021 01:50




Physics, 31.10.2021 02:00

Mathematics, 31.10.2021 02:00

History, 31.10.2021 02:00

English, 31.10.2021 02:00

Biology, 31.10.2021 02:00



Mathematics, 31.10.2021 02:00

English, 31.10.2021 02:00

Mathematics, 31.10.2021 02:00

Mathematics, 31.10.2021 02:00

Mathematics, 31.10.2021 02:00


Health, 31.10.2021 02:00