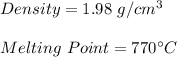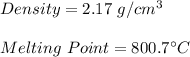Chemistry, 16.04.2020 20:55 andrewjarrah05
A soft silvery metal with a melting point of 63.5`C reacts with chlorine gas. The substance formed by the reaction is a white crystalline solid with a density of 1.98 g/cm3. Is the new substance sodium chloride? Explain.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3


Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
A soft silvery metal with a melting point of 63.5`C reacts with chlorine gas. The substance formed b...
Questions

Mathematics, 23.02.2021 07:20

Mathematics, 23.02.2021 07:20

Biology, 23.02.2021 07:20

Biology, 23.02.2021 07:20

Mathematics, 23.02.2021 07:20




Biology, 23.02.2021 07:20



History, 23.02.2021 07:20

Mathematics, 23.02.2021 07:20

World Languages, 23.02.2021 07:20


Mathematics, 23.02.2021 07:20

Mathematics, 23.02.2021 07:20


Medicine, 23.02.2021 07:20

History, 23.02.2021 07:20