

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Determine the total pressure of gas mixture the contains oxygen, nitrogen, and helium if the partial...
Questions




Geography, 06.01.2021 02:00

Chemistry, 06.01.2021 02:00


Mathematics, 06.01.2021 02:00


Mathematics, 06.01.2021 02:00

Mathematics, 06.01.2021 02:00




Chemistry, 06.01.2021 02:00




Mathematics, 06.01.2021 02:00

Mathematics, 06.01.2021 02:00

Health, 06.01.2021 02:00

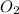 = 0.197 atm
= 0.197 atm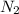 = 0.461 atm
= 0.461 atm

