Chemistry, 16.04.2020 09:35 emmaguentherp3hjd3
A can of coke contains 25 mL of carbon dioxide gas at 100kPa. If you take it on a hike up Mount Everest and the pressure decreases to 50 kPa, what will the new volume of the carbon dioxide gas in your coke can be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
A can of coke contains 25 mL of carbon dioxide gas at 100kPa. If you take it on a hike up Mount Ever...
Questions

Geography, 09.10.2019 01:30







Mathematics, 09.10.2019 01:30



Biology, 09.10.2019 01:30




Mathematics, 09.10.2019 01:30

Mathematics, 09.10.2019 01:30


Mathematics, 09.10.2019 01:30


English, 09.10.2019 01:30

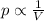
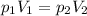
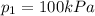 is the initial pressure of the gas in the coke
is the initial pressure of the gas in the coke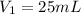 is the initial volume
is the initial volume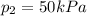 is the final pressure
is the final pressure